american academy of pediatrics covid vaccine myocarditis
It was made in the same way as other widely used vaccines including certain types of flu hepatitis and whooping cough vaccines. CDC has provided guidance regarding evaluation and management of myocarditis after mRNA COVID-19 vaccine https.

11 Things To Know About Covid Vaccines And Kids Hearts
The AAP offers professional services to help build and manage your career.

. This is the first clinical practice guideline from the American Academy of Pediatrics that specifically applies to patients who have experienced an apparent life-threatening event ALTE. The COVID-19 vaccine is our best hope for ending the current pandemic. While the goal will be to administer vaccine to as many people as possible as quickly as possible vaccine supply will be limited initially.
COVID-19 vaccine is currently authorized for some adolescents and will likely be authorized for adolescents 12 years of age and older soon. Below are common questions and answers related to implementation of COVID-19 vaccine in your practice. Below are answers to some of the most common questions.
A supply of updated COVID-19 vaccine boosters the first to specifically target the more-contagious omicron variant of the virus is slowly trickling into pharmacies across Wisconsin. While children are as likely to get COVID-19 as adults kids are less likely to become severely ill. The Journal of Pediatrics is an international peer-reviewed journal that advances pediatric research and serves as a practical guide for pediatricians who manage health and diagnose and treat disorders in infants children and adolescentsThe Journal publishes original work based on standards of excellence and expert review.
The definition of child case is based on varying age ranges reported across states see report Appendix for details and links to all data sources. Myocarditis and Pericarditis. Last updated 62222.
The known risks of COVID-19 illness and its related possibly severe complications such as long-term health problems hospitalization and even death far outweigh the potential risks of having a rare adverse reaction to vaccination including. The American Academy of Pediatrics has released a new policy that highlights vision symptoms in children and adolescents with concussion. The first two cases were discovered independently in the United.
Policy Statements Clinical Reports Clinical Practice Guidelines and Technical Reports are now in one location. The American College of Obstetricians and Gynecologists ACOG recommends that all eligible persons aged 6 months and older including pregnant and lactating individuals receive a COVID-19 vaccine or vaccine series. This clinical practice guideline has 3 objectives.
First it recommends the replacement of the term ALTE with a new term brief resolved unexplained event BRUE. Where can I find guidance on which dose they should receive. There were no cases of vaccine-associated myocarditis in the trials.
On December 18 2020 FDA issued an Emergency Use Authorization for Moderna COVID-19 vaccine to prevent. The Novavax vaccine is the newest COVID-19 vaccine. Evidence about the safety and effectiveness of COVID-19 vaccination during pregnancy has been growing.
Since the pandemic began. Novavax has less real-world safety data than the other COVID-19 vaccines because it is new. My child is on the cusp edge of an age group for which a different COVID-19 vaccine formulation is recommended.
These data suggest that the benefits of receiving a COVID-19 vaccine outweigh any known or potential risks of vaccination during pregnancy. PedJobs the official job board. Christine Oshansky Biomedical Advanced Research and Development Authority.
Trials in children 6 months to 11 years have demonstrated that these vaccines given at lower doses elicit neutralizing immune responses comparable to. Archived COVID-19 Vaccination Schedules. Acute COVID-19 severity does not necessarily predict subsequent or ongoing signs or symptoms.
Dit is de 3e versie van de uitvoeringsrichtlijn COVID-19-vaccinatie voor professionals die geldig is vanaf de start van de herhaalvaccinatiecampagne in het najaar van 2022. De vorige versies van deze richtlijn zijn niet meer actueel en gearchiveerd. Center for Vaccine Development and Global Health University of Maryland School of Medicine.
There were no cases of vaccine-associated myocarditis in the trials. Sean OLeary American Academy of Pediatrics. CDC COVID-19 Response Team Allergic Reactions Including Anaphylaxis After Receipt of the First Dose of Moderna COVID-19 Vaccine United States December 21 2020-January 10 2021 MMWR Morb Mortal Wkly Rep.
Messenger RNA mRNA and Novavax COVID-19 vaccines are preferred over the Johnson JohnsonJanssen. The Journal seeks to publish high. As a new vaccine there are many questions that pediatricians and patientsfamilies have.
Learn more about the American Academy of Pediatrics including our mission leadership and. In this QA of commonly asked questions Dr. When will the COVID-19.
However myocarditis has been documented even in people with COVID-19 who were asymptomatic or had mild infections. The 2009 swine flu pandemic caused by the H1N1 influenza virus and declared by the World Health Organization WHO from June 2009 to August 2010 is the third recent flu pandemic involving the H1N1 virus the first being the 19181920 Spanish flu pandemic and the second being the 1977 Russian flu. Learn more from the CDC about this type of vaccine.
However some children with COVID-19 need to be hospitalized treated in the intensive care. COVID-19 vaccine safety in people with rheumatologic diseases April 2022. Kids under 12 ages 5-11 years are now able to receive the COVID-19 vaccine.
I am concerned about my childs risk of myocarditis and pericarditis from vaccination. Getting a COVID-19 vaccine can protect you from severe illness from COVID-19. The precise risk is uncertain but is expected to be lower than that seen in.
CDC and the American Academy of Pediatrics AAP recommend every child continues to receive recommended vaccinations during the COVID-19 pandemic. As of June 2022 the US Food and Drug Administration FDA has authorized BNT162b2 Pfizer COVID-19 vaccine and mRNA-1273 Moderna COVID-19 vaccine for use in children 6 months and older. The American Academy of Pediatrics has released a new policy to assist health care professionals and organizations in efforts to prevent sexual abuse of children in health care settings and to.
The precise risk is uncertain but is expected to be lower. Why should I consider vaccinating my child. Zie hiervoor paragraaf 22.
Angela Dangvu a pediatrician in the CHOC Primary Care Network helps parents know what to expect when getting their younger children vaccinatedThis QA is also available in Spanish and Vietnamese. The American Academy of Pediatrics and the Childrens Hospital Association are collaborating to collect and share all publicly available data from states on child COVID-19 cases. Children represent about 19 of all reported COVID-19 cases in the US.
Federal health officialsincluding those with the American Academy of Pediatricshave advised that heart inflammation myocarditis and pericarditis is an extremely rare side effect of the COVID-19 vaccine. Wijzigingen ten opzichte van vorige versie. On June 17 2022 the Food and Drug Administration FDA issued Emergency Use Authorization EUA amendments for the mRNA-1273 Moderna COVID-19 vaccine for use in children aged 6 months5 years administered as 2 doses 25 µg 025 mL each 4 weeks apart and BNT162b2 Pfizer-BioNTech COVID-19 vaccine for use in children aged 6 months4.
AAP Policy brings together a constellation of policy documents from the American Academy of Pediatrics. Up to 50 of children and adolescents might have COVID-19 with no symptoms. Recent literature has reported a much lower incidence of myocarditis 05 to 3 than earlier in the pandemic.
Look for ways to include content related to COVID-19 and vaccine science into your lessons where appropriate.

Moderna S Covid Vaccine For Teens Awaits Ok As Regulators Review Heart Inflammation Risk
Post Vaccine Myocarditis In Young People Is Rare And Usually Mild Study Confirms American Heart Association
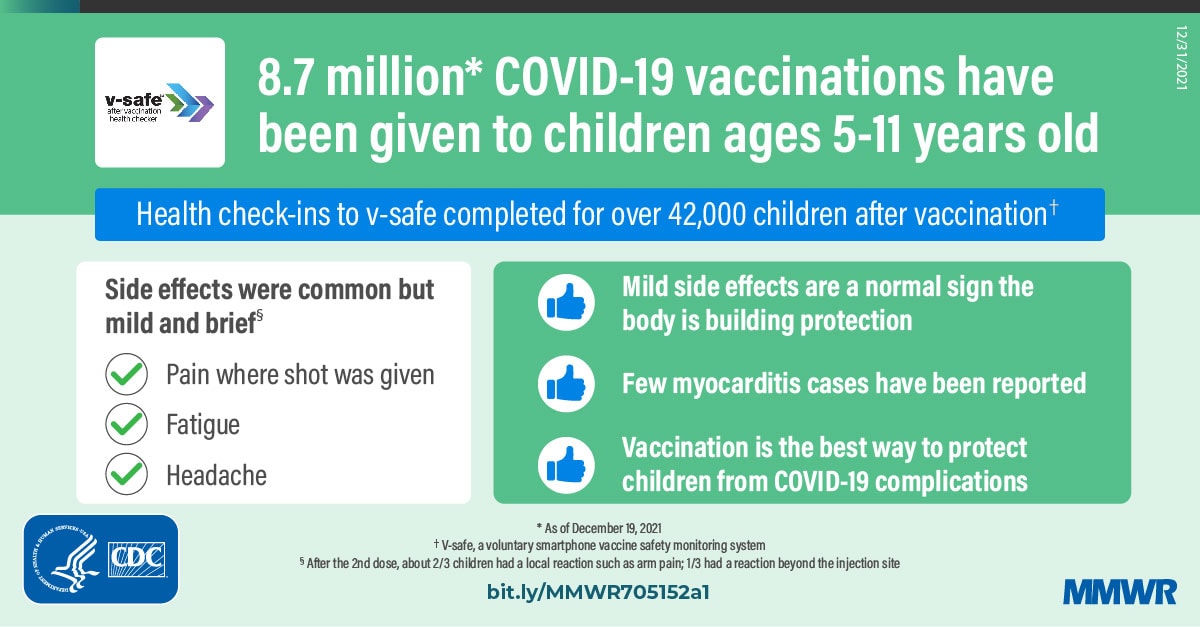
Covid 19 Vaccine Safety In Children Aged 5 11 Years United States November 3 December 19 2021 Mmwr

Heart Issues More Common After Covid Infection Than Vaccination Cdc Reports
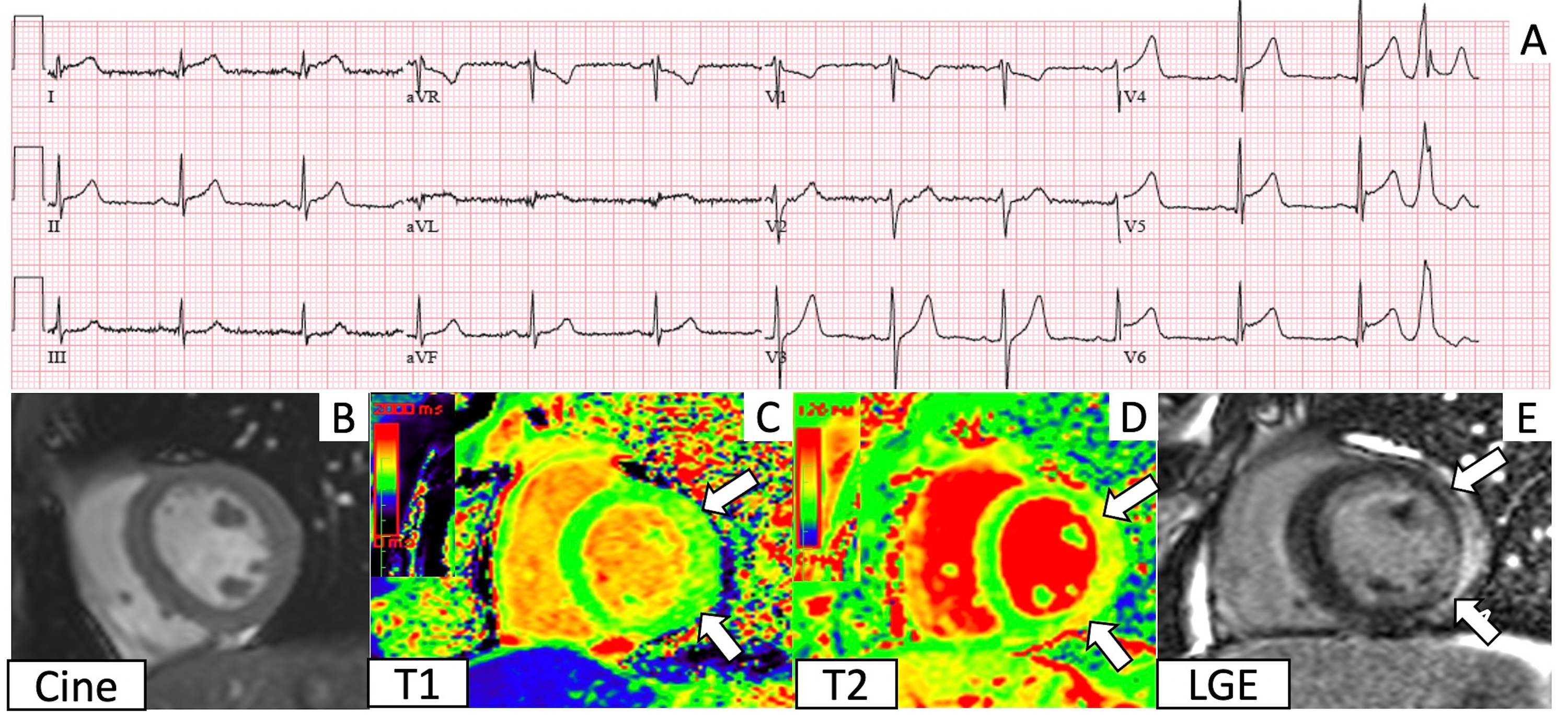
Cureus Myocarditis And Other Cardiovascular Complications Of The Mrna Based Covid 19 Vaccines

Covid 19 Vaccine Westport Ct Village Pediatrics

Considering Mandatory Covid 19 Vaccination Policies For Students

Addressing The Use Of Covid 19 Vaccines In Children Youtube

Covid 19 Vaccine Uptake Toolkit Alabama Chapter Of The American Academy Of Pediatrics
What A Pediatrician Wants Concerned Parents To Know About Covid Vaccine Musc Charleston Sc

Young People Recover Quickly From Rare Myocarditis Side Effect Of Covid 19 Vaccine American Heart Association
Live Updates Covid 19 In Kids Goodrx

Covid 19 Vaccine And Risk For Heart Inflammation In Youth What Parents Need To Know

Myocardial Injury Pattern At Mri In Covid 19 Vaccine Associated Myocarditis Radiology

Daily Dose Pediatricians And Cardiologists Covid 19 Vaccine Benefits Far Outweigh Myocarditis Risk

A Systematic Review And Meta Analysis Of The Association Between Sars Cov 2 Vaccination And Myocarditis Or Pericarditis American Journal Of Preventive Medicine
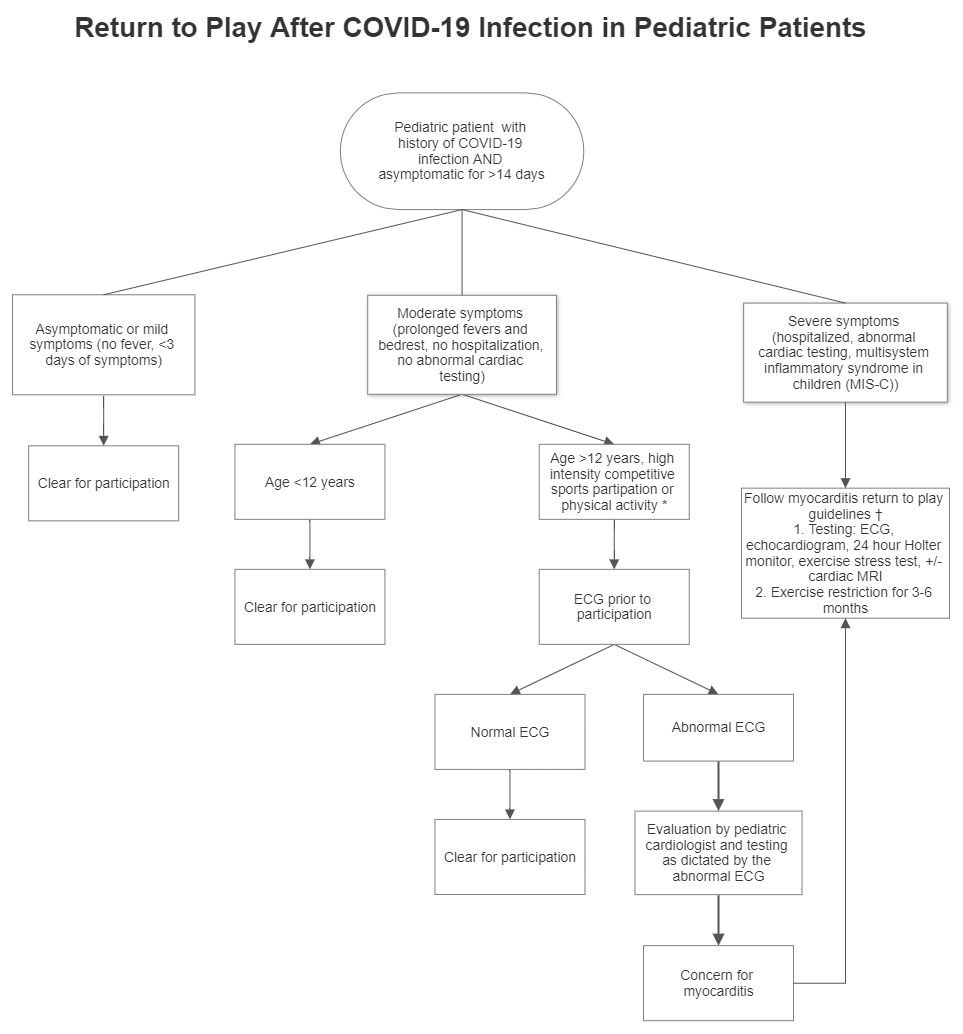
Returning To Play After Coronavirus Infection Pediatric Cardiologists Perspective American College Of Cardiology

Myocarditis And Pericarditis Weighing The Risks Of These Rare Side Effects Oregon Health News

Sars Cov 2 Mrna Vaccination Associated Myocarditis In Children Ages 12 17 A Stratified National Database Analysis Medrxiv